Catalizadores mecanizados
10.07.2018
One of the most spectacular scientific advances of the last decades has been the connection of molecular fragments through the "mechanical link". In mechanically interlocked molecules, the different components are connected thanks to their topology, in the same way for example as the links that are part of a chain. There are no direct connections (covalent bonds) between them, but nevertheless they cannot be separated without breaking their structure. This new type of chemical bond has some unique properties, in particular it allows the movement of one component of the final structure with respect to the others. Some of the pioneers of the mechanical bond have taken advantage of these properties to build artificial molecular machines capable of carrying out complex functions, such as exerting mechanical forces or synthesizing peptides with a specific sequence, imitating natural molecular machines such as myosin or ribosome, respectively. The relevance of this field was underlined with the award of the Nobel Prize in Chemistry in 2016 to Stoddart, Sauvage and Feringa for the design of artificial molecular machinery. Both Stoddart and Sauvage base their designs on the properties of the mechanical link.
Carbon nanotubes are tubes formed by graphene sheets rolled in on themselves, their physical properties make them one of the most attractive nanomaterials. For example, they are stronger than steel and can be semiconductors, like silicon, on which all current electronics are based.
Until now, carbon nanotubes have been combined with molecules via very strong bonds (covalent bonds) that lead to very stable compounds. Such connection, however, implies a change in the structure of the nanotube and therefore in its properties. It would be analogous to nailing an advertisement to a post using a thumbtack: the union is strong, but it leaves a hole in both the advertisement and the post. Weak non-covalent forces have also been used, which keep the structure of the nanotubes intact, but typically yield kinetically unstable compounds. The comparison in this case would be to tape the advert to the post. Neither the advertisement nor the post are damaged, but the union is much weaker.
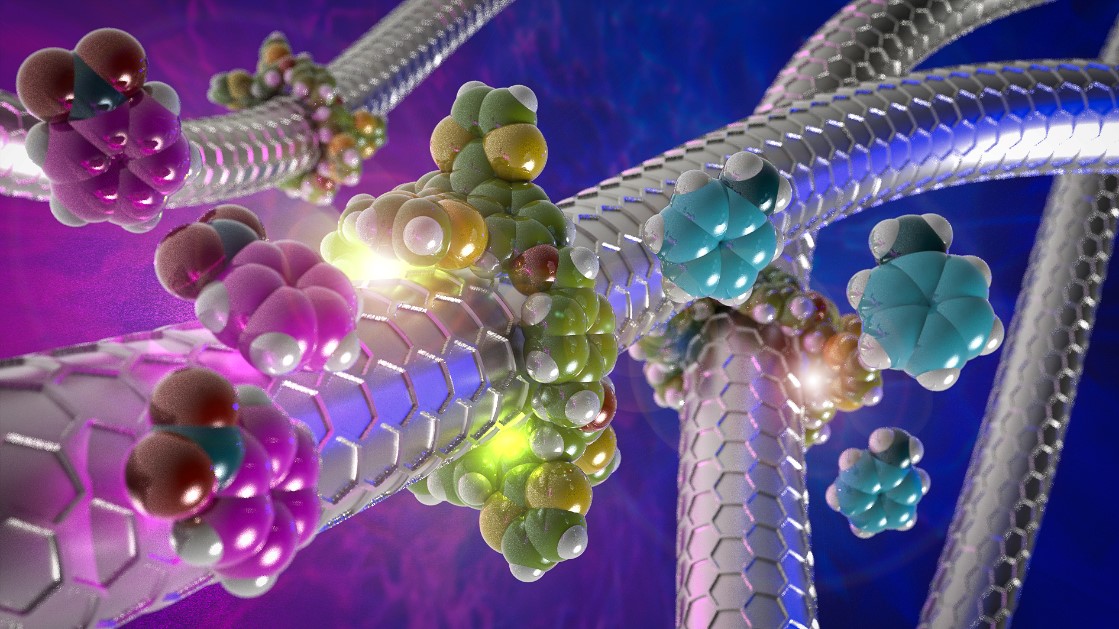
The research group led by Emilio M. Pérez at IMDEA Nanociencia (Madrid) has developed methods for the chemical modification of carbon nanotubes by mechanical bonding, the first example of mechanically interlocked carbon nanotubes (MINTs). This type of compounds are as stable as covalent compounds, but at the same time as respectful of the initial structure as the non-covalent compounds. In this case, imagine a cylindrical advertisement through which the post is threaded: the link is very strong, because we cannot separate the ad without breaking it, but it does not leave holes in the post or in the advertisement. In addition, we can move the ad up and down the length of the pole, or rotate it, to look at it from the angle that suits us best.
In results published today in Nature Communications, Emilio Pérez's group describes how to build mechanized catalysts with control over their catalytic activity. Carbon nanotubes are often very good catalysts in many relevant chemical processes, since all of their atoms are surface atoms. However, controlling their catalytic activity is not easy. In contrast, Nature devotes a significant amount of resources and applies a multitude of strategies (alosterism, chemical modification, direct competition for the active site, compartmentalization, etc.) to increase or decrease the catalytic activity of enzymes.
In the strategy described by the IMDEA Nanociencia group, the rings around the tube are used to modify the electronic properties of the nanotubes, which in turn results in a regulation of their catalytic activity, making them better or worse catalysts. This new control method presents a unique combination of the properties observed in natural regulation strategies: the link between the effectors (the macrocycles) and the catalyst (the nanotubes) is noncovalent, yet stable thanks to the mechanical link, and its effect is remote but not allosteric, since it does not change the structure of the active site (the surface of the nanotube).
Reference:
M.Blanco et al. Positive and negative regulation of carbon nanotube catalysts through encapsulation within macrocycles. Nat. Comm. 2018, DOI: 10.1038/s41467-018-05183-8
|
Contact: Twitter: @IMDEA_Nano, @emilioperezlab
Facebook: @IMDEANanociencia
|
Download