Research

Our group has interests in three main research lines: carbon nanotubes, 2D materials, and supramolecular chemistry.
Carbon nanotubes:
Among 1D nanomaterials, single-walled carbon nanotubes (SWNTs) are adorned with a particularly attractive set of physical properties. The combination of high electric and thermal conductivity, outstanding strength and stiffness, and a finite band-gap, make them suitable for a wealth of applications, from electronics to biology. At a time where SWNTs are past their hype, it is worth reminding that the advances in synthesis and purification technologies allow working with samples purer than ever. In Pulickel Ajayan’s words “now is the most interesting time to work on carbon nanotubes” (Chem. Eng. News, 2015, 93, 10-15).
Mechanically interlocked molecules (MIMs) are best known for claiming two thirds of the 2016 Nobel Prize in Chemistry, in particular those awarded to Profs. Sir J. Fraser Stoddart and Jean-Pierre Sauvage. MIMs consist of two or more separate components which are not connected by chemical (i.e. covalent) bonds. Examples of MIMs are rotaxanes, where one or more macrocycles are trapped onto a linear component (thread) by bulky substituents at its ends (stoppers) that prevent dissociation, and catenanes, where two or more macrocycles are interlocked as links in a chain. These structures are true molecules, as each component is intrinsically linked to the other through a mechanical bond, which prevents dissociation without cleavage of one or more covalent bonds. They are therefore fundamentally different from supramolecular species, where equilibrium between bound and unbound states always takes place
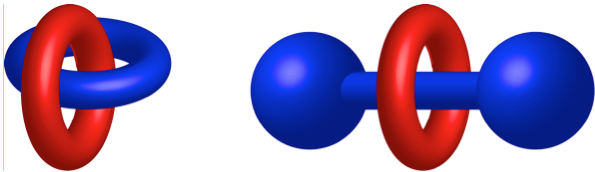
Cartoons showing the structure of a [2]catenane and a [2]rotaxane.
We have developed a general strategy for the synthesis of rotaxane-type derivatives of carbon nanotubes, the first example of mechanically interlocked derivatives of SWNTs which we call MINTs (Angew. Chem. Int. Ed., 2014, 53, 5394-5400, Highlighted in Chem&Eng News, 2014, 92, 31; Chem. Soc. Rev. 2019, 48, 5016-5032). In the key rotaxane-forming step, we employ U-shaped macrocycle precursors equipped with two recognition units (i.e. two molecular fragments that like to “stick” to the nanotube) and terminated with bisalkenes that are closed around the nanotubes through ring-closing metathesis (RCM). The formation of MINTs does not involve modification of the sp2 C-C conjugated structure of the nanotubes, from which all their amazing properties emerge. Furthermore, because the SWNTs are too long for the macrocycle to find their tips and “fall off”, the mechanical link in MINTs is as kinetically stable as a covalent bond, but without messing with the native structure of the SWNTs.
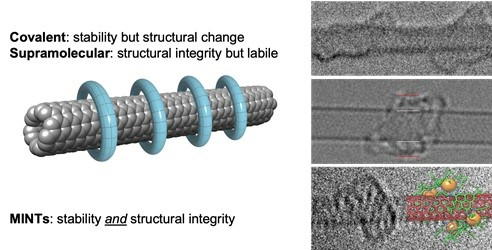
Left: Scheme of the idealized structure of MINTs and its main features compared to classic covalent and supramolecular chemistries of SWNTs. Right: Examples of HR-TEM pictures of MINTs as described in Angew. Chem. Int. Ed., 2014, 53, 5394-5400; Chem. Sci., 2018, 9, 6779-6784; and Angew. Chem. Int. Ed., 2022, 61, e202208189 (top to bottom).
We know that the electronic properties of the SWNTs are affected differently than in a classical supramolecular modification (Nanoscale, 2016, 8, 9254-9264). Moreover, the characteristics of the mechanical bond make MINTs potentially useful. For example, we know that MINTs are better at reinforcing polymers than pristine SWNTs, and way better than classic supramolecular complexes with the exact same chemical composition! (ACS Nano, 2016, 10, 8012-8018). MINTs are also promising in another classic field of application of SWNTs: catalysis. For instance, the macrocycles can be used to tune the catalytic properties of the tubes (Nat. Commun., 2018, 9, 2671; Chem. Sci., 2022, 13, 9706-9712) or the mechanical bond can be used as a stable link between SWNTs and catalytically active molecules (ACS Appl. Mater. Interfaces, 2020, 12, 32615-32621). Along the same lines, the MINT strategy can also be used to place and address single-molecule magnets within devices (J. Am. Chem. Soc., 2021, 143, 21286-21293).
And this is only the beginning… So watch this space!
2D materials:
The isolation of graphene was recognized with the Nobel Prize in Physics 2010, awarded jointly to Andre Geim and Konstantin Novoselov. Graphene is a strictly 2D layer of carbon atoms arranged in a honeycomb structure, and it is the thinnest, strongest and most conductive material known. It was isolated by mechanical cleavage from graphite using scotch tape! (seriously, watch).
The same strategy can be applied to other layered materials in which atoms in-plane are bound through strong covalent bonds and layers are held together by weak van der Waals interactions. Since the discovery of graphene, many other bidimensional materials have been isolated and characterized. We now have a pretty big collection, spanning the whole range of band-gaps from hexagonal boron nitride (h-BN) an insulator (band-gap ca. 5-6 eV), to transition metal dichalcogenides (TMDCs), which are semiconductors with band-gaps in the vis-NIR region (1-2 eV) to black phosphorus (band-gap 0.3-2 eV). With an ever-expanding toolbox of materials and properties, bidimensional materials offer the promise of a technological revolution.
While our friends the physicists have been busy studying the properties of these amazing materials, the chemists were doing what they do: make things and modify things. In the making part, the chemists developed new methods of production, by liquid-phase exfoliation (LPE). Basically, you immerse the bulk material in a solvent that can get between the weakly bound layers, and separate them, usually using ultrasound irradiation. This way you can obtain much larger quantities of bidimensional material than through mechanical exfoliation, and you obtain it in a very convenient “ink” form. Our main advance in the LPE of TMDCs is obtaining single-layer enriched suspensions in a single step, without purification, by using carefully controlled conditions (2D Materials, 2016, 3, 035014).
The covalent chemistry of 2D materials is very interesting, because it is very challenging. All atoms look the same, and are typically not very reactive, so it is difficult to do chemistry on them, and even more difficult to do chemistry selectively on some of the atoms. We have visited both of those challenges. For example, most developments in the chemistry of TMDCs focused in the more reactive 1T polytypes (metallic), but there was a need for a reliable method of functionalization of the more inert 2H, semiconducting, polytypes. We described how to decorate 2H-MoS2 and WS2 covalently with maleimides, using thiol-ene “click” chemistry (J. Am. Chem. Soc., 2019, 141, 3767-3771). This is type of chemistry is orthogonal to most other functional groups and very robust, so we expect it to allow for the modification of transition-metal dichalcogenides á la carte. With regards to the selectivity, when Bielawski, Ruoff and co-workers reported the functionalization of graphene selectively at areas with higher local curvature (see their article: Chem. Commun., 2013, 49, 677), we immediately related their results to the corrugated graphene over Ru that is routinely grown by the group of Amadeo L. Vázquez de Parga and Rodolfo Miranda. After a series of failed experiments due to overfunctionalization, we serendipitously discovered that graphene was reacting with the solvent! A little more tweaking lead to the first method for covalent functionalization of graphene with 98% atomic selectivity (Nano Lett., 2016, 16, 355-361).
We are also interested in attaching molecules to other 2D materials using weaker, noncovalent forces. For example, we have literally dropped organic dyes (perylene bisimides and porphyrins) on top of MoS2-based photodetectors. The functionalization results in a dramatic enhancement of the photoresponse and responsivity to visible light of up to three orders of magnitude. Remarkably, the process is fully reversible and reproducible (Chem. Commun., 2016, 52, 14365-14368).
Stacking one 2D material on top of another is a particularly interesting way to make new ultrathin materials, taking the best from each material. These “nanosandwhiches” of 2D materials are called “van der Waals heterostructures”, as the materials are contacted through dispersion-type noncovalent forces. These van der Waals heterostructures are made one-by-one, which is painfully slow and has other disadvantages. For example, it is nearly impossible to align the crystal lattices of the materials perfectly, and very often you include impurities between the 2D material layers. The lack of control over the interface of the two materials in terms of electronic communication, chemical nature or interlayer distance hampers the construction of robust multi-purpose devices.In collaboration with Enrique Burzurí, we have connected covalently layers of 2D materials: MoS2 and graphene (Nat. Chem., 2022, 14, 695–700). We use the tools of synthetic chemistry to sew several flakes of MoS2 to single-layer graphene devices, using a bifunctional molecule with two anchor points. The results show that the final electronic properties of the heterostructure are dominated by the molecular interface! These experiments show the power of the chemical approach to build MoS2-graphene heterostructures beyond van der Waals. The covalent connection brings an additional lever to tune the final properties of nanodevices beyond the intrinsic properties of the materials, and has the potential for facile high-throughput homologation.
In yet another collaboration, in this case with Andres Castellanos-Gomez and J. J. Palacios, we have shown that there is another very interesting way to obtain van der Waals heterostructures: let Nature make them for you! Franckeite is a naturally occurring mineral of the family of the sulfosalts that is composed of alternating layers of PbS and SnS2 that have really cool properties (small band-gap, air-stable, etc.). We have exfoliated it both mechanically and through LPE, characterized it, and made several prototype devices (Nat. Commun., 2017, 8, 14409).
Animation showing the simultaneous fabrication of several covalently connected MoS2-graphene devices.
Supramolecular chemistry:
A deep understanding of weak noncovalent interactions is of key for all our other research interests. So we also like to spend time developing tools that help us understand how molecules interact with other molecules, or with nanomaterials.To study supramolecular interactions, chemists measure association constants, basically, a number that indicates how strong the noncovalent interactions are, so it allows us to compare different interactions. Well, despite more than a decade of research in supramolecular chemistry of SWNTs, there was no standard method for the quantification of their noncovalent chemistry in solution/suspension. As supramolecular chemists, this was a challenge that excited us. We managed to solve it by realizing that you actually don’t need to know the molar concentration of SWNTs to extract accurate association constants. It is enough to know the concentrations of free and bound host. Well, d’oh! To test the method, we measured binding constants between five different hosts and two types of SWNTs in four solvents -yes, a nice big data sample, that’s the way we roll. We determined numeric values of Ka from 1 M-1 (very very small) to 104 M-1 (rather large). See how we did it here, Chem. Sci., 2015, 6, 7008-7014.
Optical tweezers (OT) are one of the most successful single-molecule force spectroscopy techniques, to the point of Arthur Ashkin being awarded with ½ of the Nobel Prize for Physics 2018, for their use to study biophysics. In a running collaboration with Borja Ibarra, we are exploring the use OT to study synthetic supramolecular systems. In our first report, we studied the force-to-break of H-bonded host-guest systems under aqueous conditions (Chem. Sci., 2017, 8, 6037-6041). Now, we are focusing on more complex, mechanically interlocked systems. In 2018, we connected a two-station molecular shuttle and observed its real time shuttling dynamics for several minutes! (Nat. Commun., 2018, 9, 4512).
We are hoping to use the combination of atomic control provided by synthetic chemistry and extreme force (ca. 1 pN) and position (ca. 1 nm) precision provided by OTs to study fundamental problems in supramolecular chemistry, such as the nature of the H-bond.